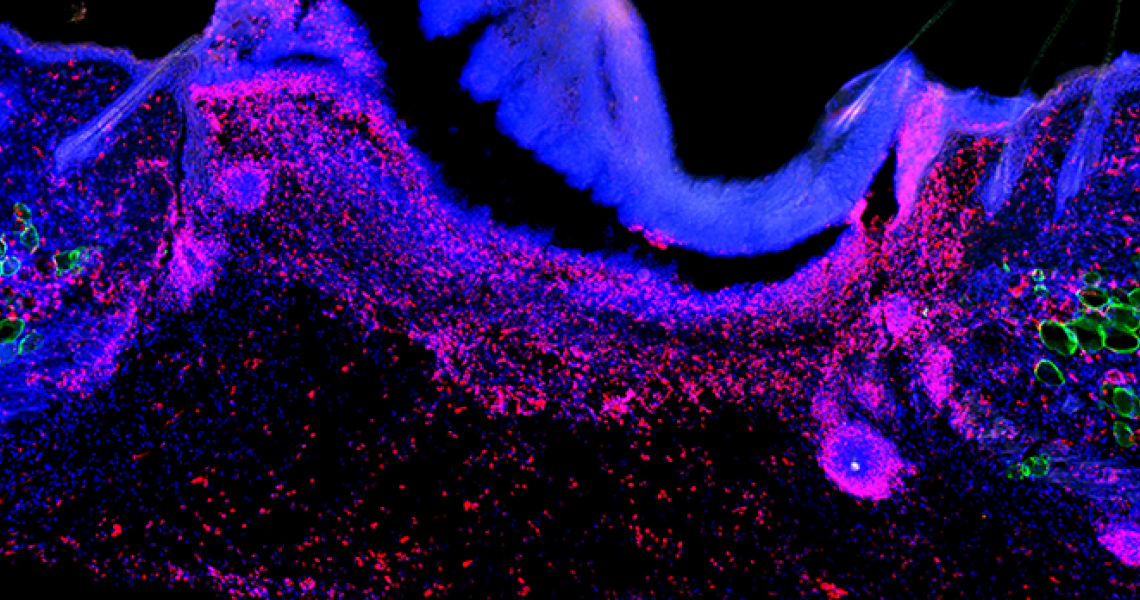
Tissue resident cells must communicate with immune cells to promote repair after injury and supports proper tissue function; however, aberrant communication is the source of tissue dysfunction associated with many pathological conditions. In the Shook Lab, we use skin as a clinically relevant tissue to investigate the function of tissue resident cells, such as fibroblasts, adipocytes and keratinocytes, during acute and chronic inflammation. Our goal is to uncover therapeutically targetable cellular and molecular mechanisms that connect to inflammation. We combine powerful unbiased “-omics” approaches with hypothesis driven genetic tools to make scientific discoveries.
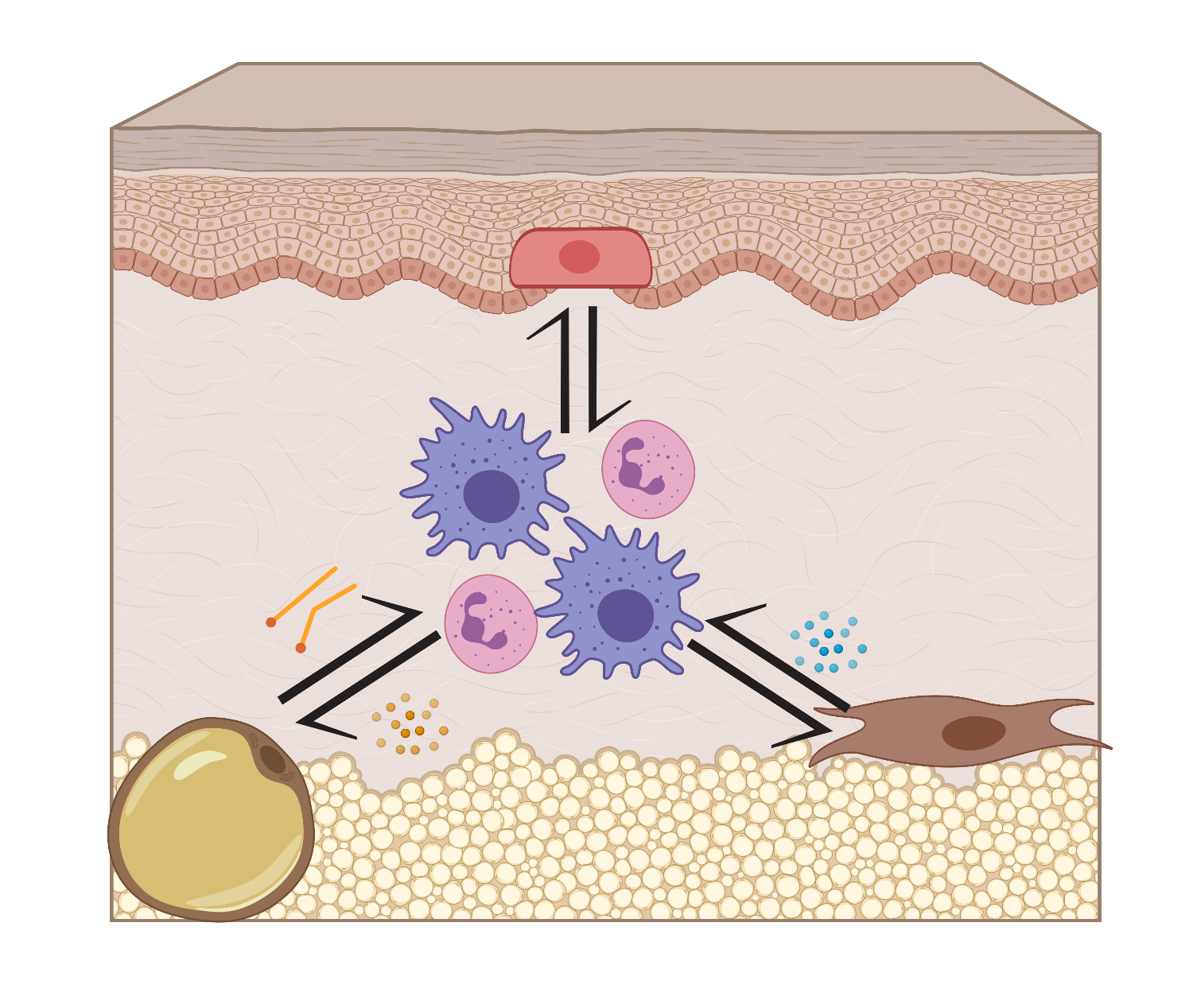
How do tissue resident cells contribute to inflammation?
The skin contains incredible cellular diversity, with dozens of unique cells making up the dermis. In particular, fibroblasts and adipocytes are two abundant cell types that support cutaneous function. In the Shook Lab, we are exploring how these cell types regulate immune cell behavior.
Fibroblasts: Due to their ability to rapidly generate a wealth of extracellular matrix molecules, fibroblasts have largely been investigated during fibrogenic conditions. However, fibroblasts can also drive inflammation. Currently, we are exploring how fibroblasts contribute to wound-induced inflammation and how inflammation alters fibroblast function.
Adipocytes: While visceral white adipose tissue adipocytes are powerful regulators of local immune cell composition and function and the mammalian body is marbled with adipose tissue, very little is known about how tissue resident adipocytes interact with the immune system during acute or chronic inflammatory responses. Free fatty acids can be utilized for energy or function as potent signaling molecules. We have previously identified that adipocyte lipolysis is required for proper macrophage inflammation after injury. We are currently exploring how adipocyte lipolysis contributes to wound-induced macrophage responses. The current line of investigation combines pharmacological and genetic animal models with in vitro analyses. These experiments will allow us to better understand how macrophage inflammation is regulated after injury to discover molecular mechanisms that can be therapeutically targeted.
How do pathological conditions alter stromal cell function?
Diabetes and aging significantly alters the behavior of fibroblasts in various tissues and adipocytes in visceral and subcutaneous white adipose depots. Some of the most profound changes in stromal cell function are increased expression of inflammation-associated genes. Given the high prevalence of impaired wound healing and comorbidity of inflammatory cutaneous diseases in diabetic patients, understanding how diabetes and aging changes dermal fibroblast and adipocyte function could lead to significant therapeutic advances. We are currently investigating how fibroblast responses and dermal adipocyte lipolysis are altered in diabetic and aged skin and how this contributes to altered inflammation.